Invited
Speaker
Old drugs with new faces: Chemical recycling of Primaquine
as a new strategy to control vivax malaria.
Paula Gomes
Portugal
Tropical and sub-tropical countries are malaria-endemic. In 2007,
the United Arab Emirates was the first formerly-endemic country since
the 1980s to be certified malaria-free, but the menace on the Middle
East keeps active. Further, global-warming and exodus of populations
from endemic to malaria-free regions may underlie the risk of malaria
worldwide spread.1,2
Plasmodium vivax is the predominant malaria parasite in Asia
and the Middle East, significantly contributing to morbidity among
people of all ages. 70 to 80 million cases of vivax occur
annually, causing major negative effects on economic productivity
at the individual and pannational levels. Outside of Africa, vivax
accounts for over 50% of cases, with 80-90% occurring in the Middle
East, Asia and Western Pacific. Also, vivax parasites develop
hypnozoites, dormant forms inhabiting host’s liver that cause
relapse, in a way perpetuating the morbidity burden.3
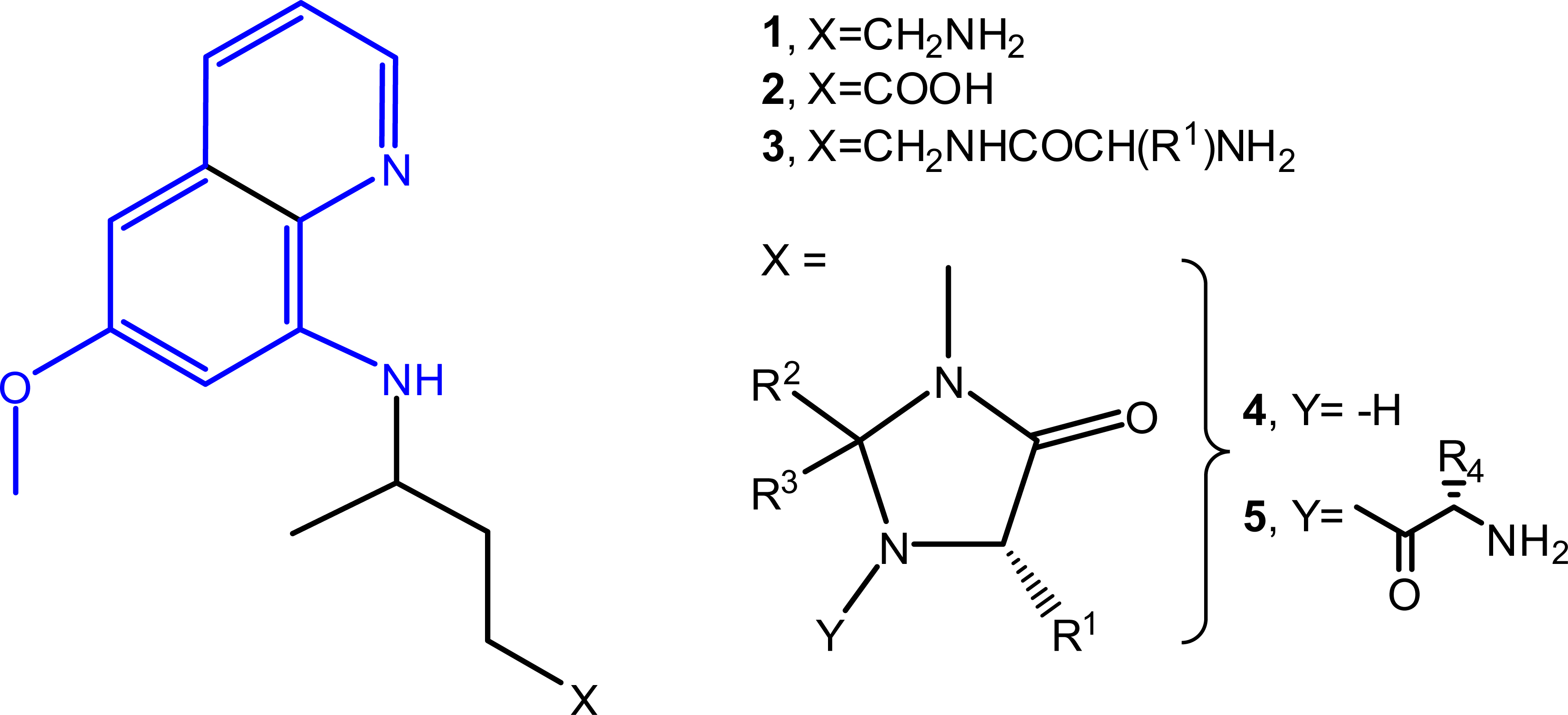
Primaquine (PQ, 1) is a ~60-year-old drug that blocks
malaria transmission from humans to mosquitoes, being also the only
available antimalarial preventing relapse.4
However, PQ has a low therapeutic index due to its metabolic conversion
into inactive carboxyprimaquine (2).5
Starting from primaquine derivatives 3,6
we created Imidazoquines (4,5)
as metabolically-stable leads, found to act against liver parasites
and block malaria transmission in vivo.7-14.
[1] WHO, Malaria Report 2008; [2]
Millet, J.P. et al. Malaria J. 2008, 7,
56; [3] Anstey, M.N. et al. Trends Parasitol.
2009, 25, 220; [4] Baird, J.K.
Clin. Infectious Dis. 2004, 39,
1336; [5] Vale, N. et al. Eur. J. Med. Chem.
2009, 44, 937; [6] Portela, MJ et al.
Pharm. Res. 1999, 16, 949; [7]
Gomes, P et al. Tetrahedron 2004, 60,
5551; [8] Araújo, MJ et al. J. Med. Chem.
2005, 48, 888. [9] Chambel, P
et al. Tetrahedron 2006, 62, 9883;
[10] Ferraz, R et al. J. Org. Chem. 2007,
72, 4189; [11] Vale, N et al. Bioorg. Med.
Chem. Lett., 2008, 18, 485; [12]
Vale, N et al. Bioorg. Med. Chem. Lett. 2008,
18, 4150; [13] Vale, N et al. Tetrahedron
2008, 64, 11144; [14] Vale, N
et al. J. Med. Chem. 2009, ahead-of-print
(doi: 10.1021/jm900738c).
|



