Poster Presenter
Comparative Study Between
Cephalexine and Cefadroxil Thermal Behaviours
Adriana Fulias, Bogdan Tita, Dumitru Tita
Romānia
In this abstract, thermoanalytical techniques were used
to study thermal behaviour of cefadroxil and cephalexine. These
substances are antibiotics from the cephalosporin family, known
as a first generation cephalosporin used to treat certain infections
caused by bacteria such as pneumonia and bone, ear, skin, and urinary
tract infections.
Differential scanning calorimetry study was performed on Netzsch
differential scanning calorimeter, model DSC–204, using aluminium
crucibles under nitrogen atmosphere, with a constant flow of 50
ml•min–1 and a heating
rate β= 5, 7, 10 and 15 K•min–1
up to a temperature of 500°C. Thermogravimetrical analysis was
performed on Perkin–Elmer DIAMOND equipment in temperature
range 25–550°C, using an air atmosphere and under dynamic
conditions in order to study the thermal stability of the active
substance.
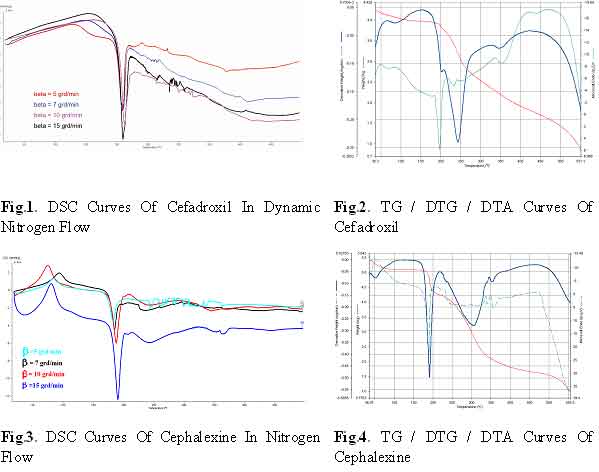
DSC curves of cefadroxil (Fig.1.) show a sharp exothermic peak at
~210°C that corresponds to melting followed by thermal decomposition.
The decomposition is defined in two exothermic stages. This is confirmed
by TG/DTG curves (Fig.2.) that indicate thermal decomposition in
the following temperature range: 191–320°C, 320–400°C
and over 400°C a slow and continuous mass loss caused by elementary
carbon formation from the previous steps, as consequence of the
rupture of the azabicyclo and phenyl aromatic rings.
In the case of cephalexine, in both air and nitrogen (Figures 3
and 4), had rather the same behaviour, a clear decomposition step
in a very narrow range, 180-200°C. The single and well developed
DTG indicates probably a single step process. The exothermic DTA,
even in nitrogen, is due to enough oxygen atoms in the molecule
for the beginning of an intramolecular oxidation. The same values
of the DTG and DTA maximum indicate a relative low exothermic effect.
|



