Sesion
Speaker
The Role of Transition Metals and Irradiation in the Action
of Anticancer Drug Camptothecin
Klaudia Jomová, Marián Valko
Slovakia
The anticancer drug camptothecin (CPT) is a plant alkaloid that is
very effective in the treatment of gastrointestinal tumours. Several
clinically important derivatives of CPT have been also synthesized;
the most recent involve topotecan and irinotecan. The anticancer activity
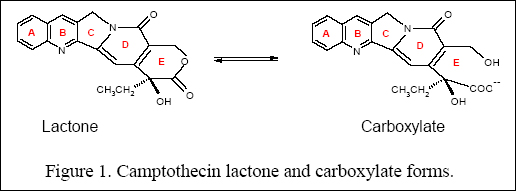
of CPT family of drugs is dependent on the maintenance of the lactone
ring in the closed form (ring E) (Figure 1).
We investigated whether the transition metal ions, namely copper could
support the efficient encapsulation of irinotecan into liposomes and
stabilize the biologically active lactone form of the drug. The 1,2-distearoyl-sn-glycero
phosphocholine/cholesterol (60:40 mol%) liposomes formulated with
350 mM copper chloride exhibited >95% irinotecan loading after
a 10 min incubation at 45 oC. The irinotecan loading has been further
supported by Cryo- Transmission Electron Microscopy. Irinotecan and
topotecan encapsulated in liposomes, were found to exist mainly in
the therapeutically active lactone form (90% and 92% of the total
drug, respectively).
In addition, we studied the photochemical properties of CPT in the
presence of various metal ions. The EPR spin trapping experiments
indicate that CPT is a promising photosensitizer and that free radicals
and singlet oxygen generated upon illumination of CPT play a key role
in DNA cleavage in cancer cells.
|



