| Chemistry
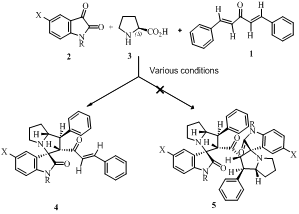
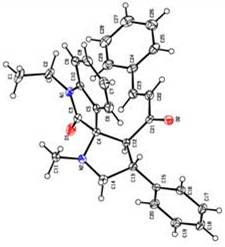
(Track) ASYMMETRIC SYNTHESIS OF NEW SPIRO-OXINDOLOPYRROLIZIDINES Abdollah Javidan, Mohammad Javad Taghizadeh and Khosrow Jadidi Department of Chemistry, Imam Hossein University, Tehran, Iran Some spiropyrrolidines are potential antileukemic and anticonvulsant agents [1] and possess antiviral [2] and local anaesthetic [3] activities. In this paper we will report asymmetric 1,3-dipolar cycloaddition reaction of the dipolarophiles 1 and azomethine ylides to give the novel spiro pyrrolo/pyrrolizidinos instead of bis- spirooxindolopyrrolizidines. Condensation of compounds 2 and 3 after decarboxylation leading to the non-stabilized azomethine ylides stereogenic centers in one step. We expected by this method a bis spirooxindolopyrrolizidine 5 and 8 prepared. But by using this strategy only diastereoisomers 4 and 7 were obtained The structures of cycloaddition products 5 were assigned by IR, 1HNMR, 13CNMR, Mass spectral data and single crystal X-ray analysis (Fig. 1).
Refrences |