Chemistry (Track)  A MODULAR APPROACH TO CHIRAL IMIDATES: A NEW CLASS OF NITROGEN-BASED CHIRAL LIGANDS AS TOOLS FOR MEDICINAL CHEMISTRY Johan Van der Eycken,Timothy Noël, Katrien Bert and Pieter Janssens Laboratory for Organic and Bioorganic Synthesis, Department of Organic Chemistry, Ghent University, Krijgslaan 281 (S.4), B-9000 Ghent, Belgium Nitrogen-containing ligands are known as cheap, readily accessible and stable alternatives for phosphane ligands [1], which are often very sensitive to air and require a multistep synthesis [2]. We wish to present a combinatorial approach to a novel type of nitrogen-based mono- and bidentate ligands [3]. These ligands are characterized by their modular structure, allowing an easy one-step synthesis by simply combining two readily variable precursors which are either commercially available, or can be reached in only a few steps: a cyclic imidate 1 and a (chiral) amine, respectively diamine. These ligands show promising results in e.g. the Cu(I)-catalyzed asymmetric aziridination of methyl cinnamate, in asymmetric diethylzinc additions to benzaldehydes, in the Pd(0)-catalyzed asymmetric allylic alkylation and amination, and in Ir(I)-based asymmetric hydrogenation of alkenes, and hence are promising tools for medicinal chemistry. 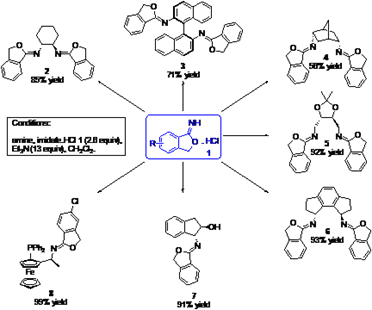 References |