CONFERENCE ABSTRACT (INVITED SPEAKER)

Stereoselective Synthesis of Bioactive Compounds (Track)
AN APPROACH TO THE SYNTHESIS OF VIOPROLIDES
Eibhlin Butler, Lucia Florentino and Eric J. Thomas
The School of Chemistry, The University of Manchester, Manchester M13 9PL, UK
Abstract
The vioprolides [1] are a group of depsipeptides that have interesting biological activity, not the least being a synergistic effect on the murine type 1 Interferon (IFN) signalling pathway leading to more than a three-fold increase in activity [2]. They also modulate the NF-κB pathway in cell-based assays [3]. These studies were carried out using a sample of natural vioprolide A but this material is extremely scarce and more material is necessary for further biological studies. To date no synthesis of a vioprolide has been reported and only very preliminary synthetic approaches have been described [4,5].
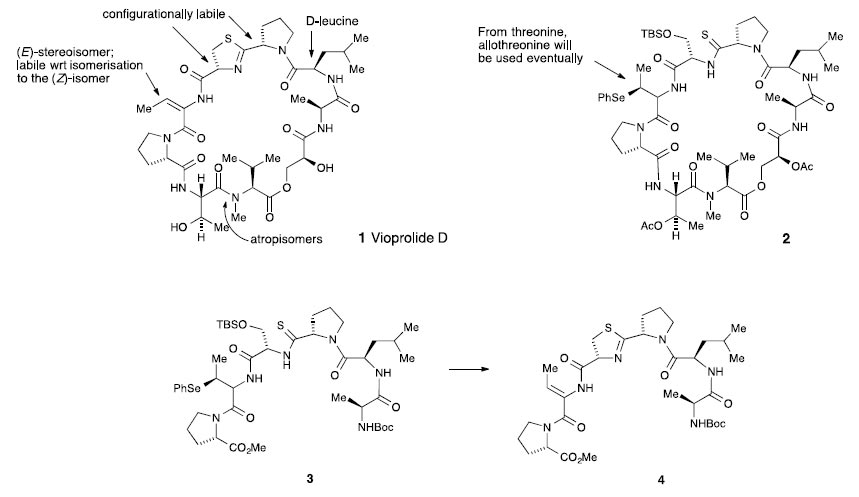
A synthetic approach to vioprolide D 1 will be discussed. To date, work has been concerned with the total synthesis of the advanced macrocyclic intermediate 2. To complete the synthesis it will be necessary to introduce the required (E)- dehydrobutyrine and thiazoline fragments. Indeed procedures have been developed for the dehydration of the modified hexapeptide 3 into the (Z)-dehydrobutyrine – thiazoline containing peptide 4. The required (E)-isomer may be obtained by isomerisation of the (Z)-isomer or by using allothreonine as the starting material. Procedures will also be developed for cleavage of the acetates to release the natural product.
REFERENCES
[1] D. Schummer, E. Forche, V. Wray, T. Domke, H. Reichenbach and G. Hofle, Leibigs Ann., 1996, 971.
[2] M. Bollati-Fogolin, M. Oggero, S. Mirazo, R. Frank, R. Kratje and W. Muller, Cells and Culture, 2010, 485.
[3] M. Bollati-Fogolin, unpublished observations.
[4] N. Chopin, F. Couty and G. Evano, Lett. Org. Chem., 2010, 7, 353.
[5] H. Liu and E. J. Thomas, Tetrahedron Letters, 2013, 54, 3150.