CONFERENCE ABSTRACT (SESSION SPEAKER)

Chemistry (Track)
HYDRAZONES AS KEY REAGENTS AND LIGANDS FOR NOVEL APPLICATIONS IN ASYMMETRIC CATALYSIS
Rosario Fernández
Departamento de Química Orgánica, Universidad de Sevilla, Sevilla, Spain
Abstract
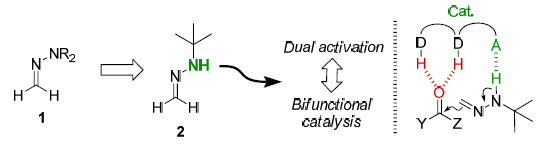
The asymmetric 1,2 addition of formyl anion equivalents to carbonyl compounds is a powerful synthetic tool that provides a direct access to functionalized carbinols. In this context, we have exploited the aza-enamine character of formaldehyde N,N-dialkyl-hydrazones (d1 synthons) [1], but the development of a catalytic. enantioselective version remained elusive. However, replacement of the N,N-dialkylamino by a N-tert-butylamino group enables a dual activation by bifunctional Hbonding organocatalysts to perform highly enantioselective additions to α-ketoesters [2], isatines [3] and α- ketophosphonates (Fig. 1) [4].
 .
.
Figure 1. Dual activation in the addition of formaldehyde t-butyl hydrazone to carbonyl compound.
On the other hand, hydrazones appear as an interesting class of useful ligands in diverse contexts. The use of C2–symmetric pyrrolidines as terminal dialkylamino groups, making the rotations around N–N bonds inconsequential, is a key design element that enabled a high enantiocontrol in a variety of reactions (Fig. 2). The use of bis-hydrazones I, phosphinohydrazones II, and pyridino-hydrazones III in a variety of asymmetric catalytic transformations [4-8] will be discussed.
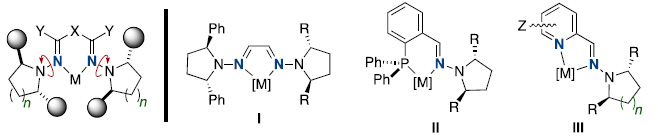
Figure 2. Hydrazone-based chiral ligands.
REFERENCES
[1] Crespo, A.; Monge, D.; Martín-Zamora, E.; Álvarez, E.; Fernández, R.; Lassaletta, J. M. J. Am. Chem. Soc. 2012, 134, 12912.
[2] Monge, D.; Crespo, A.; Martín, E.; Álvarez, E.; Fernández, R.; Lassaletta, J. M. Chem. Eur. J. 2013, 19, 8421.
[3] Serrano, I.; Monge, D.; Álvarez, E.; Fernández, R.; Lassaletta, J. M. Chem. Commun. 2015, 51, 4077
[4] Lassaletta, J. M.; Alcarazo, M.; Fernández, R. Chem. Commun. 2004, 298.
[5] Bermejo, A.; Ros, A.; Fernández, R.; Lassaletta, J. M. J. Am. Chem. Soc. 2008, 130, 15798.
[6] Ros, A.; Estepa, B.; Bermejo, A.; Álvarez, E.; Fernández, R.; Lassaletta, J. M. J. Org. Chem. 2012, 77, 4740.
[7] Egger, L.; Tortoreto, C.; Achard, T.; Monge, D.; Ros, A.; Fernández, R.; Lassaletta, J. M.; Lacour, J. Adv. Synth. Catal. 2015,
357, 3325.
[8] Álvarez-Casao, Y.; Monge, D.; Álvarez, E.; Fernandez, R.; Lassaletta, J.M. Org. Lett. 2015, 17, 5104.