CONFERENCE ABSTRACT (SESSION SPEAKER)

Chemistry (Track)
INNOVATIVE STRATEGIES FOR THE ASYMMETRIC SYNTHESIS OF (HETERO)BIARYLS
José M. Lassaletta
Instituto de Investigaciones Químicas (CSIC-US). Americo Vespucio 49, 41092 Sevilla, Spain
Abstract
The asymmetric Suzuki−Miyaura cross-coupling is a challenging reaction, requiring catalysts that combine a high activity to achieve reactions with hindered coupling partners and a geometry able to drive enantiocontrolled reactions. Our contribution in this field centered on the use of hydrazonebased N/N and P/N ligands for the coupling of unfunctionalyzed and formyl(acyl)-substituted electrophiles, respectively [1]. However, these approaches failed for the coupling of heterocyclic derivatives, probably due to problems associated with the coordination ability of the substrates, the stability of the required organometallics, and/or the lower configurational stability of the desired heterobiaryls. In this lecture, new strategies based in dynamic kinetic resolution (DKR) of borylated heterobiaryls [2] (via cross-coupling) and dynamic kinetic asymmetric Suzuki- Miyaura couplings (DYKAT) of heterobiaryl triflates [3] will be discussed.
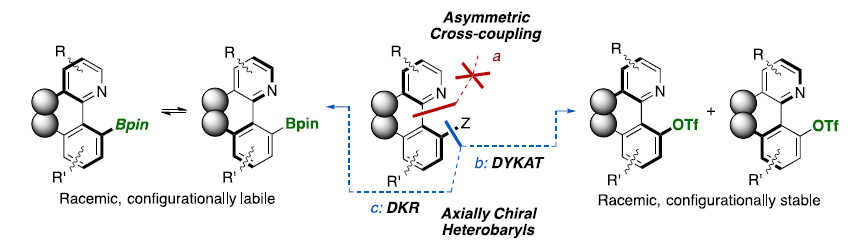 .
.
REFERENCES
[1] (a)Bermejo, A.; Ros, A.; Fernández, R.; Lassaletta, J. M. J. Am. Chem. Soc. 2008, 130, 15798; (b) Ros, A.; Estepa, B.;
Bermejo, A.; Álvarez, E.; Fernández, R.; Lassaletta, J. M. J. Org. Chem. 2012, 77, 4740.
[2] Available via Ir-catalyzed directed ortho-borylation (a) Ros, A.; Estepa, B.; López-Rodríguez, R.; Álvarez, E.; Fernández, R.;
Lassaletta, J. M. Angew. Chem. Int. Ed. 2011, 50, 11724; (b) Ros, A.; López-Rodríguez, R.; Estepa, B.; Álvarez, E.;
Fernández, R.; Lassaletta, J. M. J. Am. Chem. Soc. 2012, 134, 4573.
[3] Ros, A.; Estepa, B.; Ramírez-Loṕez, P.; Álvarez, E.; Fernández, R.; Lassaletta, J. M. J. Am. Chem. Soc. 2013, 135, 15730.